Short summary on Abel et al. 2022. Skull sutures and cranial mechanics in the Permian reptile Captorhinus aguti and the evolution of the temporal region in early amniotes. Frontiers in Ecology & Evolution.
Me, Yannick Pommery (Université de Bourgogne), David P. Ford (University of the Witwatersrand), Daisuke Koyabu (University of Tsukuba), and Ingmar Werneburg (Senckenberg Centre, University of Tübingen) published a new paper on the morphology of the skull sutures of the early amniote Captorhinus aguti and their macroevolutionary implications in Frontiers in Ecology & Evolution. Next to a general description of the sutures, we roughly reconstruct the arrangement of the jaw adductor musculature and discuss the stress distribution within the skulls together with the possibility of intracranial movements. Lastly, we illustrate how possible precursors for temporal openings can be already observed in an early "anapsid" taxon like C. aguti. This study forms part of my doctoral thesis.
What is it all about?
- Most extant tetrapods possess openings in their temporal skull region. This differs from the majority of their Paleozoic ancestors in which the temporal region is fully covered by dermal bones.
- In amniotes (mammals, birds and other reptiles), temporal openings likely formed due to adaptations of a stronger external jaw adductor musculature. A heterogeneous stress regime during biting probably led to the strengthening of loaded areas (i.e., temporal bars) and loss of functionally "unneeded" areas in the temporal dermatocranium (i.e., the formation of temporal openings).
- However, this is difficult to test in vivo since extant taxa with a similarly covered temporal region (e.g., sea turtles) are highly derived compared to Paleozoic taxa. Hence, inferences have to be drawn from fossil material.
- If the scenario described above is correct, we should be able to find evidence of this heterogeneous stress regime in the skull of a Paleozic amniote without temporal openings ("Anapsida").
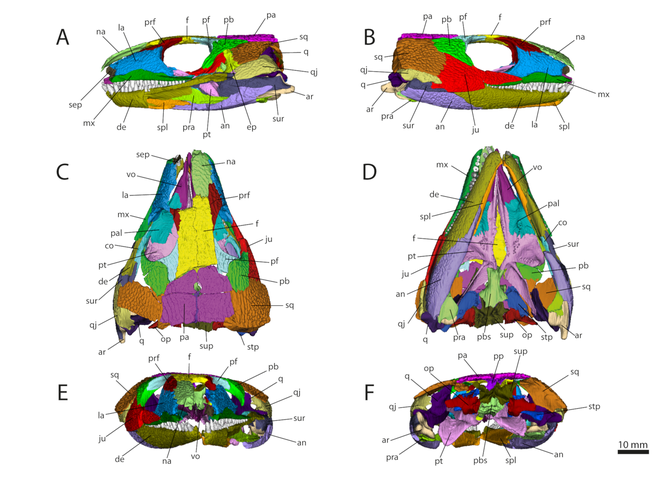
3D model generated based on the microCIT data of OMNH 44816 in left lateral (A), right lateral (B), dorsal (C), ventral (D), anterior
(E), and posterior view (F). Abbreviations: an, angular; ar, articular; co, coronoid; de, dentary; ep, epipterygoid; f, frontal; ju, jugal; la, lacrimal; mx,
maxilla; na, nasal; op, opisthotic; pa, parietal; pal, palatine; pb, postorbital; pf, postfrontal; pp, postparietal; pra, prearticular; prf, prefrontal; pbs, parabasisphenoid; pt, pterygoid; q,
quadrate; qj, quadratojugal; sep, septomaxilla; spl, splenial; sq, squamosal; stp, stapes; sup, supraoccipital; sur, surangular; vo, vomer. From Abel et al. (2022).
What did we do?
- To test this, we used the microCT-scan of a skull of the early amniote Captorhinus aguti (Eureptilia, Captorhinidae) from the early Permian of Oklahoma to generate a 3D model of each preserved skull bone.
- The specimen (OMNH 44816) is stored at the Sam Nobles Oklahoma Museum of Natural History and has been scanned at the CT facility of the University of Texas in 2017. The bones were segmented and measured in Avizo and visualized in MorphoDig.
- Next to describing the sutures of the whole dermatocranium and its contacts with the neuro- and viscerocranium, we drew inferences from extant tetrapods to roughly reconstruct the jaw adductor musculature of an early amniote.
- Based on this, we discussed to possibilities of cranial kinesis and elasticity in C. aguti and how stress was likely distributed within the temporal region.
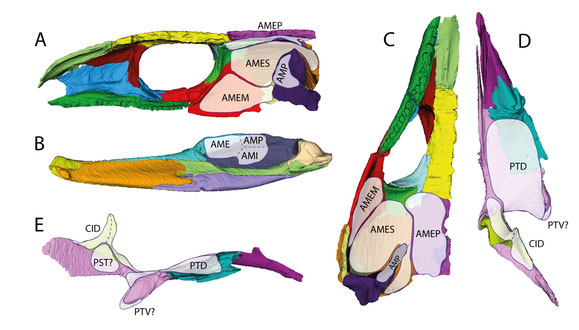
Reconstructed distribution of the jaw adductor musculature of an early amniote as exemplified by C. aguti. (A) right half of the
cranium in medial view; (B) right mandibular ramus in medial view; (C) right half of the cranium in ventral view; (D) right palate in dorsal view; (E) right palate in lateral view. Abbreviations:
AME, adductor mandibulae externus; AMEM, adductor mandibulae externus medialis; AMEP, adductor mandibulae externus profundus; AMES, adductor mandibulae externus
superficialis; AMI, adductor mandibulae internus; AMP, adductor mandibulae posterior; CID, constrictor internus dorsalis; PST, pseudotemporalis; PTD,
pterygoideus dorsalis; PTV, pterygoideus ventralis. From Abel et al. (2022).
What did we find?
- We identified eight different suture morphotypes in the dermatocranium of OMNH 44816, ranging from simple butting joints to complex interdigitations.
- In the temporal region, overlapping sutures prevail. Often, the bones are also interdigitating at their external surfaces. These are likely adaptations to withstand compressional stress acting on the temporal dermatocranium during biting.
- While there runs an interdigitated contact line dorsally from the skull roof through the "cheek", the interdigitation sequence pauses at the jugal-squamosal-postorbital intersection.
-
At this intersection, the dermal armor is also thinner than in the rest of the "cheek", suggesting that this area was less loaded during biting.
- Additionally, the parietal is laterally distinctly overlapping the squamosal and anteriorly overlaps/interdigitates with the frontal and prefrontal, but exhibits only a simple butt joint with the postorbital. Likely indicating a less loaded area.
- Some parts like the connection between skull roof and "cheek", as well as the anterior palate were likely relatively elastic. However, C. aguti was likely not capable of any true cranial kinesis like it can be observed in many extant tetrapods.
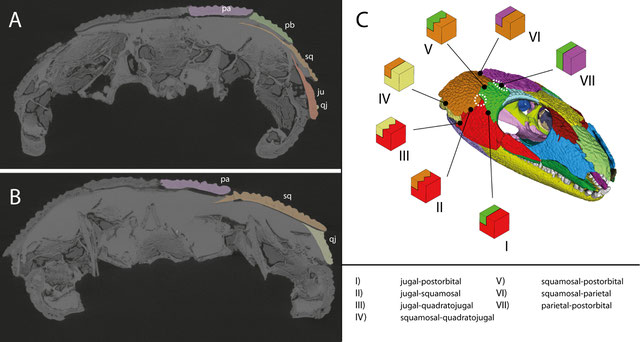
MicroCT images of the anterior (A) and posterior (B) temporal region in OMNH 44816 in posterior view, highlighting the overlapping sutures of the dermal bones. (C) Suture morphotypes present in the temporal region of OMNH 44816. Dashed circles indicate potentially weak areas. From Abel et al. (2022).
What are the implications?
- There are simpler and thinner areas in the temporal dermatocranium, which is in accordance with the expected heterogeneous stress regime.
-
The thin jugal-squamosal-postorbital intersection fits previous observations and may represent a possible pre-stage for an
infratemporal fenestra. However, in many taxa the infratemporal fenestra also incorporates the quadratojugal.
- Especially in parareptiles, the infratemporal fenestra forms at the jugal-squamosal-quadratojugal intersection, which wasn't weakened in C. aguti but probably in their parareptilian ancestor due to differences in stress distribution.
- Three-bone-intersections might be generally prone to form openings (see Frazzetta 1968). The common fenestra incorporating jugal, squamosal, postorbital, and quadratojugal might form by a fusion of two smaller fenestrae that form in the mentioned three-bone-intersections (2 and 3 in the figure below). Indeed, two infratemporal fenestrae can appear simultaneously within both intersections as evident by some ophiacodontids (Romer & Price 1940).
- Also, the simple postorbital-parietal contact corresponds to the position of the diapsid supratemporal fenestra.
- C. aguti has its limitations as it lived 30 million years after the first amniotes and already exhibited various derived traits, especially in its jaw apparatus. Also, there are no known captorhinids with temporal openings, even though the jaw adductors distinctly increased in later forms.
- Nevertheless, preliminary observations in other early "anapsids" like the diapsid-relative Protorothyris archeri seem to mostly confirm the pattern reported for C. aguti.
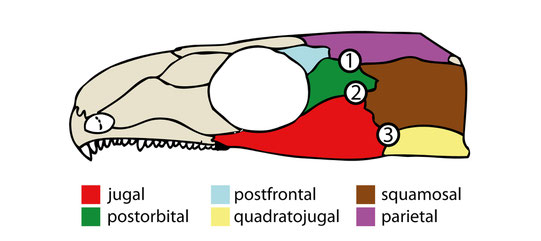
Cranium of C. aguti after Fox & Bowman (1966), highlighting the dermal bones of the temporal region. Numbers indicate possible origination sites for temporal openings in early amniotes. (1) and (2) represent also "weak" areas observed in OMNH 44816. From Abel et al. (2022).
References
- Fox, R.C., & Bowman, M.C. (1966). Osteology and relationships of Captorhinus aguti (Cope) (Reptilia: Captorhinomorpha). The University of Kansas Paleontological Contributions Vertebrata 11, 1–79.
- Frazzetta, T.H. (1968). Adaptive problems and possibilities in the temporal fenestration of tetrapod skulls. Journal of Morphology 125, 145–157.
- Romer, A.S., & Price, L.I. (1940). Review of the Pelycosauria. Special Paper – Geological Society of America 28, 1–538.
Do you have any questions about this study? Feel free to contact me.